Программа AutoCAD дает прекрасную возможность профессиональному художнику создать полноценные и невероятно реалистичные 3D-модели даже больших …
Подробнее »-
Забор живая изгородь: как вырастить и сформировать
Смотреть на обычные заборы, какими бы ни были они красивыми, удовольствие сомнительное. Для того чтобы …
Подробнее » -
Забор из металлического штакетника (евроштакетника) своими руками
-
Забор из профлиста своими руками: пошаговый фотоотчет
-
Забор из сварной 3D сетки гиттер (Gitter)
-
Как выбрать и установить электрозамок на калитку
-
Перепланировка-2023: запреты, порядок согласования и наказание за нарушения
В арендованной квартире не сделаешь серьезный ремонт без согласия хозяев. А в своей можно развернуться? …
Подробнее » -
Резка керамогранита: 5 инструментов для самостоятельной работы, подробные инструкции и видео
-
Перепланировка ванной: 10 можно и нельзя, 5 законных проектов
-
Какие обои лучше выбрать для дома: сравнение виниловых, флизелиновых и бумажных обоев
-
Шумоизоляция стен в квартире: типы, нужные материалы, инструкции по самостоятельной сборке
-
Твердотопливные котлы: от выбора до инноваций
С развитием индустрии и технологий систем отопления, многообразие доступных решений для обогрева домов и промышленных …
Подробнее » -
Инфракрасные обогреватели: принцип работы и преимущества для современного дома
-
Тепловые насосы для отопления частных домов
-
Отопление нового поколения: что нужно знать о электрических котлах
-
Самостоятельное создание водопровода на даче: шаг к комфорту и удобству
-
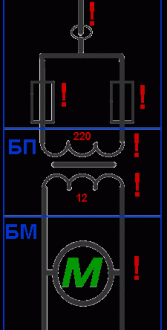
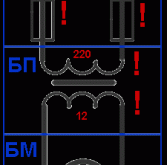
Использование поблочно-последовательного метода при поиске неисправностей в электрических схемах
Что такое блок? Некое устройство или участок схемы, выбранный нами, имеющее так называемые «вход» и …
Подробнее » -
Полезные советы по поиску неисправностей при ремонте электрооборудования
-
Передача электроэнергии по одному проводу — выдумка или реальность?
-
Как определить неисправность тиристоров
-
Как найти правильного электрика для замены электропроводки в доме
-
Как продумать функциональные спальные места?
Дизайн детской для мальчика и девочки вместе предполагает оформление не только зонированного пространства, но и …
Подробнее » -
Детская комната для девочки и мальчика
-
Освещение в детской
-
Дополняющие элементы и аксессуары
-
Принципы выбора террасной доски: Руководство для заботливых владельцев
 Ваш прораб | Профессиональная ремонт Профессиональная ремонт
Ваш прораб | Профессиональная ремонт Профессиональная ремонт